1. Discussion
This is essential to ensure individuals make an informed choice about their contraception options and can give informed consent. These may be undertaken face-to-face, via telephone or virtual appointment, or by self-assessment and signposting to patient resources. Women can be encouraged to watch an eight minute information film produced by Lothian Sexual Health available at https://www.lothiansexualhealth.scot/contraception/iud-ius/
2. When can a Cu-IUD be inserted
A Cu-IUD can be inserted at any time during the menstrual cycle providing that pregnancy can be reasonably excluded (see Box 1). Recommendations for starting or switching to a Cu-IUD can be found in Table 2 and Table 3.
The Cu-IUD can be used for EC if inserted within 5 days of the first episode of UPSI that cycle, or within 5 days of the earliest expected date of ovulation (see emergency contraception protocol)
Contraception After Pregnancy | CoSRH

Table 2: Starting Cu-IUD (no recent hormonal contraception) [from CoSRH Clinical Guideline: Intrauterine contraception (March 2023)]
|
Current Situation
|
Timing of insertion of LNG-IUD |
Additional Precautions required |
|
No recent hormonal contraception and no recent pregnancy
|
Any time in a natural menstrual cycle if reasonably certain the individual is not pregnant* or at risk of pregnancy (unless qualifies for pregnancy use as EC) |
No |
|
Cu-IUD within licensed duration of use
|
Any time |
Ideally abstain/use condoms for 7 days prior to change in case new device cannot be inserted unless criteria for EC insertion are met |
|
Cu-IUD past licensed duration of use
|
Any time in a natural menstrual if reasonably certain the individual is not pregnant* or at risk of pregnancy (unless qualifies for use as EC) |
No |
|
Post partum (vaginal birth or Caesarian section, breastfeeding or non-breast feeding) |
Within 48 hours after childbirth or from 4 weeks after childbirth if it is reasonably certain the individual is not pregnant* or at risk of pregnancy (unless criteria for use as EC apply) |
No |
|
Following abortion of miscarriage |
Post-surgical abortion or surgical management of miscarriage: ideally insert at the time of the procedure
Post-medical abortion or miscarriage: IUC can be inserted any time after expulsion of pregnancy
|
No |
|
Following use of oral emergency contraception |
Within the first 5 days (120 hours) following first UPSI in a natural menstrual cycle or within 5 days after the earliest estimated day of ovulation |
No additional precautions required
|
|
If there has been UPSI in this natural menstrual cycle that occurred >5 days ago AND it is >5 days after the earliest estimated date of ovulation (or date of ovulation cannot be estimated), a Cu-IUD cannot be inserted until pregnancy can be excluded by a high-sensitivity pregnancy test taken ≥21 days after last UPSI |
Condoms or bridging contraception until Cu-IUD can be inserted |
UPSI (unprotected sexual intercourse)
*See Box 1 for how to exclude pregnancy.
Table 3: Switching to Cu-IUD from a hormonal contraceptive method
|
Current Situation
|
Timing of insertion |
Additional Precautions required |
|
CHC use |
At any time if CHC has been used correctly (or criteria for use as EC are met) |
No |
|
POP (traditional, desogestrel or drospirenone
|
At any time if POP has been used correctly (or criteria for use as EC are met) |
No |
|
ENG implant within 3 years after insertion
|
Any time |
No |
|
ENG implant in situ for 3-4 years |
Any time if PT negative |
No Repeat PT 21days after last UPSI |
|
ENG implant in situ for >4 years and no UPSI in the last 21 days |
Any time if PT negative |
No |
|
ENG implant in situ for >4 years and UPSI in the last 21 days |
If PT negative AND all UPSI that has taken place in the last 21 days was within the last 5 days, Cu-IUD can be inserted as EC |
No |
|
Cu-IUD cannot be inserted if any UPSI occurred between 5 and 21 days ago |
Consider PT and EC. Bridge with alternative contraception until pregnancy can be excluded by a high sensitivity PT taken ≥21 days after last UPSI |
Table 3: Switching to Cu-IUD from a hormonal contraceptive method (contd)
|
Current Situation
|
Timing of insertion |
Additional Precautions required |
|
Progestogen-only injectable (DMPA) ≤14 weeks post-injection
|
Any time |
No |
|
Progestogen-only injectable (DMPA) >14 weeks post-injection and no UPSI since 14 weeks
|
Any time |
No |
|
Progestogen-only injectable (DMPA) >14 weeks post-injection AND UPSI since 14 weeks post-injection, all of which took place ≥21 days ago
|
Any time if PT negative |
No |
|
Progestogen-only injectable (DMPA) >14 weeks post-injection AND UPSI since 14 weeks post-injection, some of which took place within the last 21 day
|
If PT negative AND all UPSI that has taken place in the last 21 days was within the last 5 days, Cu-IUD can be inserted as EC |
No |
|
Cu-IUD cannot be inserted if any UPSI occurred between 5 and 21 days ago |
Consider PT and EC. Bridge with alternative contraception until pregnancy can be excluded by a high-sensitivity PT taken ≥21 days after last UPSI |
Table 3: Switching to Cu-IUD from a hormonal contraceptive method (contd)
|
Current Situation
|
Timing of insertion |
Additional Precautions required |
|
52 mg LNG-IUD in situ for < 8 years OR 19.5 mg LNG-IUD in situ for < 5 yrs OR 13.5 mg LNG-IUD in situ for < 3 yrs |
Any time |
No Ideally abstain/use condoms for 7 days prior to change in case new device can’t be inserted |
|
52 mg LNG-IUD in situ for >8 yrs and no UPSI within the last 21 days
OR
19.5 mg LNG-IUD in situ for >5 yrs and no UPSI within the last 21 days
OR
13.5 mg LNG-IUD in situ for >3 yrs and no UPSI within the last 21 days
|
Any time if PT negative on day of replacement |
No |
|
52 mg LNG-IUD in situ for >8 years† and UPSI within the last 21 days OR 19.5 mg LNG-IUD in situ for >5 years and UPSI within the last 21 days OR 13.5 mg LNG-IUD in situ for >3 years and UPSI within the last 21 days
|
If PT negative AND all UPSI that has taken place in the last 21 days was within the last 5 days, Cu-IUD can be inserted as EC |
No |
CHC, combined hormonal contraception; DMPA, depot medroxyprogesterone acetate; DRSP, drospirenone; ENG, etonogestrel; HFI, hormone-free interval; IUC, intrauterine contraception; LNG-IUD, levonorgestrel intrauterine device; POP, progestogen-only pill; PT, pregnancy test; UPSI, unprotected sexual intercourse. †Recommendations for the 52 mg LNG-IUD insertion relate to devices inserted before age 45 years.
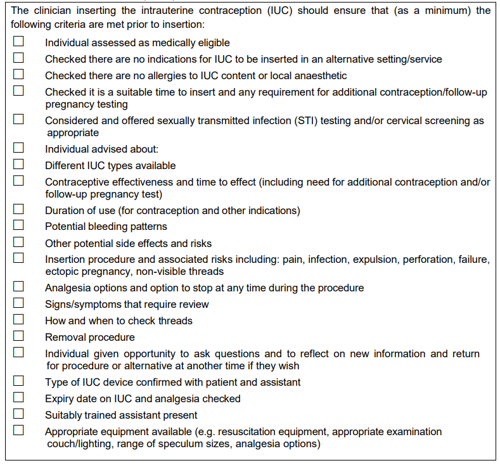
Insertion checklist
Intrauterine contraception pre-insertion checklist for the minimum criteria that should be met prior to insertion.
Safe Cu-IUD Insertion
Training: Clinicians offering Cu-IUD insertion should hold the COSRH Letter of Competence in Intrauterine Techniques. Immediate postpartum intrauterine contraception (PPIUC) technique is different to standard Cu-IUD insertion and should only be performed by those who have trained in this technique.
Assistants and Chaperones: A chaperone should be offered for all intimate examinations. The chaperone’s role is to support the patient. An appropriately trained assistant should be present during all cervical instrumentation procedures. The assistant can also fill the role of a chaperone if trained. The assistant should support the individual during the Cu-IUD procedure and monitor the patient for any signs of pain or distress.
Check the device has not expired: If an expired device is inadvertently inserted, inform the individual of the error and offer the option of retaining the device or having it removed and replaced. The expiry date relates to the microbiological sterility of the device. Risk of infection from loss of microbiological sterility could well be lower than the risk of infection if the device is replaced again. Manage the error according to local clinical governance policies.
Pain associated with Cu-IUD insertion
Cu-IUD insertions can cause mild-to-moderate pain or discomfort. Analgesia options should be discussed, offered and documented. NSAIDs such as ibuprofen can reduce pain after Cu-IUD insertion.
Emergency management for problems at IUD insertion
Cu-IUD insertion can trigger a vasovagal response. Drugs and equipment required for
resuscitation must be available, accessible, clearly labelled, adequately maintained and their location known to all staff. Follow locally agreed risk management policies for the treatment of emergencies.
Documentation
Clinicians inserting or removing Cu-IUDs should document the procedure and consultation in line with local policy and protocol and notify (where applicable and with consent) other relevant healthcare providers (e.g. primary care) of the type of device, date of insertion and recommended duration of use.
