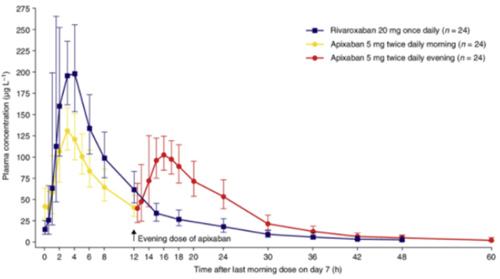
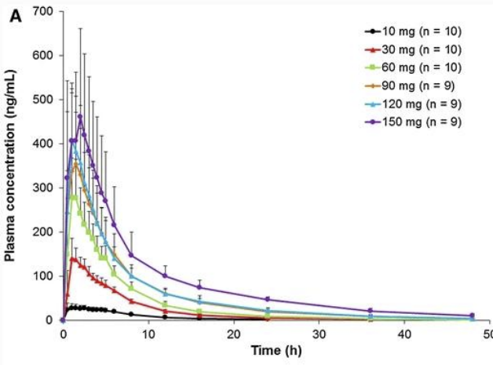
From the half-life data and concentration-time graphs, it is apparent that with normal renal function, by 24 hrs post dose (~2 half-lives) the plasma concentration and therefore clinical anticoagulation is significantly lower than peak levels.
Surgery/anaesthetic procedures can be classified as either low risk/consequence of bleeding or high risk/consequence of bleeding.
Low risk surgery/procedures would include those where direct pressure can stop bleeding with low risk of harm e.g. body surface surgery or fascia iliaca block. High risk surgery would include: major body cavity surgery, vascular surgery, and spinal/neurosurgery. High risk anaesthetic procedures would include central neuraxial blockade.
For low-risk procedures, omission of a single dose and proceeding 24 hours post dose is acceptable. For high-risk procedures, 48 hours should elapse4.
For ease of management/to allow regional anaesthesia, elective patients should therefore be advised to take their last dose of Rivaroxaban, Apixiban or Edoxaban 48 hours preoperatively.
When restarting therapeutic dosing, maximal anticoagulation can be expected 4 hours post dose.