Bleeding
Give information about expected bleeding patterns as they can change with LNG-IUD use. Although unscheduled bleeding may be caused by the LNG-IUD itself, other causes (e.g. pregnancy, infection, pathology) should be considered and investigated in line with CoSRH Clinical Guideline: Problematic Bleeding with Hormonal Contraception.
Options for HMB include:
Tranexamic acid, NSAIDs or a 3-month trial of COC or switch to LNG-IUD (if medically eligible).
New Onset Pelvic Pain
This should be assessed, and pregnancy excluded. Causes may or may not be related to the LNG-IUD. A clinical history and physical examination will identify the differential diagnoses and guide the investigation and management.
Table Three: Possible causes if new onset pelvic pain [from CoSRH Clinical Guideline: Intrauterine contraception (March 2023)]
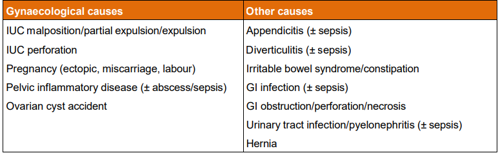
Pregnancy
The risk of any pregnancy, including ectopic pregnancy, during use of LNG-IUD is very low. Risk of ectopic pregnancy during use of LNG-IUD is lower than using no contraception. However, among pregnancies that occur with LNG-IUD in situ, the proportion that is ectopic is greater than among pregnancies occurring without LNG-IUD in situ.
A previous ectopic pregnancy is not a contraindication to use of IUC (UKMEC1).
If someone with an LNG-IUD in situ has a positive pregnancy test, follow local assessment pathways
Pregnancy less than 12 weeks gestation and threads visible: removal may improve pregnancy outcome
Pregnancy after 12 weeks gestation: refer to obstetric team
Infection
The risk of PID appears to increase in the first 3 weeks after LNG-IUD insertion but overall, the risk is very low (<1% of all IUD users). Instrumentation of the uterus can lead to ascending infection and PID.
If symptoms are suggestive of PID manage as per PID guideline (see guideline, insert link)
In mild-to-moderate cases, the Cu-IUD can remain in situ if there is improvement over the following 2-3 days. If not, recommend removing the Cu-IUD, considering pregnancy risk from the previous 7 days. Discuss alternative contraception, the need for EC and follow-up pregnancy testing. Delay further IUD insertion until antibiotic treatment has been completed and all signs and symptoms have resolved.
Candida & Bacterial vaginosis (BV): symptomatic, recurrent, confirmed VVC/BV not controlled by standard management may require switch to an alternative method of contraception.
Actinomycosis and presence of actinomyces-like organisms (ALO): Incidental findings of ALO are rare now that liquid-based cytology (LBC) and/or primary human papillomavirus (HPV) testing are used for cervical screening.
Malposition
If malposition is suspected clinically or detected on a scan refer to senior clinician
Advise use of an alternative method of contraception meantime
Perforation
Overall risk of approximately 1–2 per 1000, greater if breastfeeding and postpartum at the time of insertion.
If identified at the time of insertion: Stop procedure: remove Cu-IUD; monitor blood pressure and pulse rate and level of discomfort until stable. Consider broad-spectrum antibiotics to reduce the risk of peritonitis. Offer alternative contraception and advise to seek review if significant pain or signs/symptoms of infection develop.
Delayed identification of perforation. Lower abdominal pain, non-visible threads or changes in bleeding could indicate uterine perforation but are non- specific.
Arrange urgent USS to locate the device. If not seen on scan, arrange a plain abdominal and pelvic X-ray. In the interim, consider EC, and offer alternative contraception.
Morbidity associated with detection and removal of an intraabdominal IUD is low but uterine perforation can involve damage to the abdominal or pelvic viscera, bladder or bowel. If confirmed perforation, refer to gynaecology.
Wait at least 6 weeks after a known or suspected uterine perforation before inserting a subsequent IUD. Refer to service with available ultrasound.

