| Patient <25kg/m2 OR Osmotic symptoms if weight loss, check ketones ?T1DM |
Patient ≥25kg/m2 | |
|
SELF-MANAGEMENT: Diet and exercise |
1 month Treat immediately if symptomatic |
3 months:
|
|
FIRST LINE: Oral monotherapy |
SULFONYLUREA (SU) |
METFORMIN (MET)
|
|
SECOND LINE: Oral dual therapy |
SU + MET |
MET + ONE of the following:
|
|
THIRD LINE: Oral triple therapy |
Not appropriate. Requires insulin initiation. |
MET + TWO of the following:
OR consider INJECTABLE THERAPY, eg:
|
|
Alternative THIRD LINE: if high CV risk or obesity |
Not appropriate. Requires insulin initiation. |
GLP-1 RA or dual GIP/GLP-1 RA: can be used with insulin, metformin and/or SGLT2 inhibitor:
|
|
INSULIN therapies |
Usually start with basal insulin at bed:
|
|
|
Notes: |
|
|
Achieving control in type 2 diabetes (Guidelines)

What's new / Latest updates
July 2025:
- GLP1 prescribing choices changed:
- First line: From liraglutide & semaglutide injection to Semaglutide injection & oral
- Second line: Dulaglutide. No change
- Third line added: Tirzepatide
- 1mg max dose of semaglutide clarified.
- Amalgamation of 4 guidelines into one:
- Achieving control in type 2 diabetes (guidelines) | Right Decisions
- Pioglitazone prescribing algorithm (Guidelines) | Right Decisions
- SGLT2 inhibitors in type 2 diabetes (T2D) (Guidelines) | Right Decisions
- Glucagon-like peptide-1 (GLP-1) analogues (Guidelines) | Right Decisions
July 2024: Pdf table transposed to TAM template.
Audience
- North NHS Highland only
- Primary and Secondary Care
- Adults only
Review diet, exercise and adherence to medication before making dose adjustments or prescribing additional therapy
Discontinue new agents if no evidence of effectiveness (ie <5.5mmol/mol improvement in HbA1c) at 3 to 6 months.
HbA1c target individualised eg:
- ≤53mmol/mol on single agent
- ≤58mmol/mol on two or more agents.
Place of therapy
Sulphonylureas and metformin
Prescribing information
|
Medication |
SU: Gliclazide |
SU: Glipizide |
Biguanide: Metformin |
|
Initiation dose |
40 to 80mg before breakfast |
2·5 to 5mg before breakfast |
500mg with breakfast for 1 week, then 500mg twice daily. |
|
Dose titration increment |
40 to 80mg |
2·5 to 5mg |
500mg to 1 gram |
|
Titration interval |
|
3 monthly |
|
|
Maximum dose |
160mg twice daily before meals |
20mg daily, as divided doses, with meals |
1 gram twice daily |
|
Treatment failure criteria |
<5·5mmol/mol reduction in HbA1c in 6 months
|
||
|
Renal impairment |
<50mL/min: initially 20 to 40mg daily monitor closely and |
<50mL/min: initially 2·5mg daily monitor closely and use with caution |
|
|
Hepatic impairment |
Reduce dose |
Withdraw, if tissue hypoxia likely |
|
|
Notes |
|
|
|
| For full prescribing information, including all cautions, contra-indications and adverse effects, see BNF. | |||
Thiazolidinedione and DPP4 inhibitors
Pioglitazone prescribing algorithm
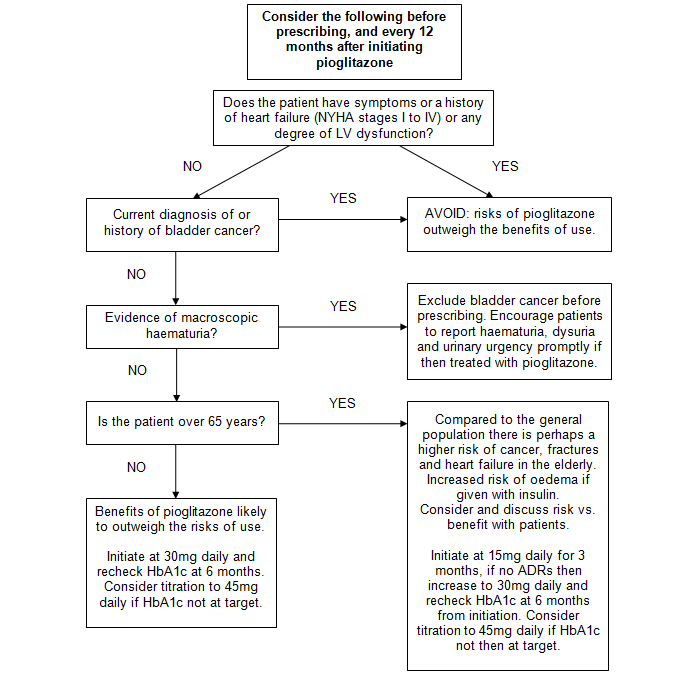
Treatment failure criteria: <5·5mmol/mol reduction in HbA1c in 6 months.
- Unless at individualised target.
- If treatment failure criteria met on maximum tolerated dose: consider withdrawal of medication, substitution or addition of another medication.
Prescribing information
|
Medication |
Thiazolidinedione: Pioglitazone |
DPP4-I: Alogliptin |
DPP4-I: Linagliptin |
|
Initiation dose |
|
25mg once daily |
5mg once daily |
|
Dose titration increment |
15mg |
N/A |
|
|
Titration interval |
|
N/A |
|
|
Maximum dose |
45mg daily |
25mg daily |
5mg daily |
|
Treatment failure criteria |
<5·5mmol/mol reduction in HbA1c in 6 months
|
||
|
Renal impairment |
Dose as in normal renal function |
≥30 to <50mL/min: <30mL/min: 6.25mg once daily |
Dose as in normal renal function |
|
Hepatic impairment |
Avoid | Avoid in severe hepatic impairment | Dose as in normal hepatic function |
|
Notes
|
|
|
|
| For full prescribing information, including all cautions, contra-indications, adverse effects, see BNF. | |||
SGLT2 inhibitors
SGLT2 inhibitors are a relatively new class of drug initially licensed for their glucose lowering effects in type 2 diabetes.
Evidence suggests they provide benefits beyond HbA1c reduction, eg, reducing risk of hospitalisation for heart failure, cardiovascular death, and progression of diabetic nephropathy.
Factors to consider when prescribing SGLT2 inhibitors in T2DM
Some agents have licenses for specific indications. The following flow chart demonstrates some of the factors to consider when considering initiation of an SGLT2 inhibitor.
Prescribing information
Sodium-glucose co-transporter 2 (SGLT2) inhibitors (Formulary)
Consideration should also be given to reducing sulfonylurea or insulin dose, if adding in an SGLT2 inhibitor, to reduce risk of hypoglycaemia.
Importantly, glucose lowering effects are dependent on adequate renal function.
- Hence SGLT2 inhibitors should NOT be started for glucose lowering if eGFR <60mL/min.
- However, they can be commenced with an eGFR between 30 to 60mL/min for renoprotection in individuals with proteinuria or in the management of heart failure with reduced ejection fraction, along with ACE inhibitor/ARB etc.
If used for these specific indications then they should be continued if eGFR drops <45mL/min, unless in the context of acute kidney injury, and can be continued if eGFR drops <30mL/min.
|
Medication |
Dapagliflozin |
Canagliflozin |
Empagliflozin |
|
Initiation dose |
10mg once daily If severe hepatic impairment: 5mg once daily |
100mg once daily |
10mg once daily Not recommended if >85 years |
|
Dose titration increment |
N/A |
To 300mg daily |
Can be increased to 25mg once daily |
|
Titration interval |
N/A |
If no side effects at 3 to 6 months |
|
|
Maximum dose |
10mg daily |
300mg Reduce to 100mg/day if eGFR falls <60mL/min |
25mg daily Reduce to 10mg if eGFR falls <60mL/min |
|
Treatment failure criteria |
<5·5mmol/mol reduction in HbA1c in 6 months, unless using for renoprotection or heart failure |
<5·5mmol/mol reduction in HbA1c in 6 months, unless using for CV benefit or heart failure |
|
|
|||
|
Renal impairment |
Can continue if eGFR <45mL/min for renoprotection or heart failure |
Can continue if eGFR <45 mL/min for renoprotection if proteinuria |
Can continue if eGFR <45mL/min for CV benefits or heart failure |
|
Hepatic impairment |
5mg daily, increase according to response |
Avoid in severe hepatic impairment |
|
|
Notes See additional guidance on prescribing for renal / cardiac disease |
|
||
| For full prescribing information, including all cautions, contra-indications and adverse effects, see BNF. | |||
GLP-1 RA & dual GIP/GLP-1 RA
Indication
- For use in individuals with type 2 diabetes and a BMI 30kg/m2 or over
- Third-line agent where as a combination therapy HbA1c is above target of 58mmol/mol
- Fourth-line agent as a combination therapy with oral antihyperglycaemics and insulin
Prescribing details
GLP-1 RA & dual GIP/GLP-1 RA (Formulary)
- FIRST LINE: Semaglutide. Increased clinical effectiveness.
- Once weekly, injectable
- Once daily, oral tablets. Alternative when subcutaneous route not suitable.
- SECOND LINE: Dulaglutide
- Once weekly, injectable. Needle is hidden, may be suitable in needle phobia.
- THIRD LINE: Tirzepatide
- Once weekly, injectable. Less cost-effective option.
|
Medication |
Semaglutide Sub-cut (Weekly) |
Semaglutide Oral (Daily) |
Dulaglutide Sub-cut (Weekly) |
Tirzepatide Sub-cut |
|
Initiation dose |
0.25mg once weekly Prescribe single prefilled 0.25mg pen |
3mg once daily Take on empty stomach 30mins before eating / drinking / other meds |
1.5mg once weekly 0.75mg weekly if monotherapy |
2.5mg once weekly |
|
Dose titration increment |
Increase to 0.5mg after 4 weeks (using 0.5mg pen) |
To 7mg, then 14mg |
1.5mg |
2.5mg |
|
Titration interval |
4 weeks |
6 months, if required |
Monthly, continue at lowest effective dose |
|
|
Maximum dose |
1mg* once weekly |
14mg daily |
4·5mg once weekly |
15mg once weekly |
|
Treatment failure criteria |
<11mmol/mol of reduction in HbA1c ±<3% weight loss in 6 months |
Weight loss <5% and/or HbA1c reduction <5mmol/mol at 6 months |
||
|
||||
|
Renal impairment |
Avoid if eGFR <15mL/min |
No dose adjustment required |
||
|
Hepatic impairment |
Avoid in severe hepatic impairment |
No dosage adjustment required |
Use with caution in severe hepatic impairment |
|
|
Notes |
Caution if diabetic retinopathy
|
NB: needle pre-attached |
Caution if diabetic retinopathy Low risk of hypo (reduce /stop SU) May reduce effectiveness of oral contraceptive pill |
|
|
||||
*Licensed max is 2mg, however the recommended maximum dose for diabetes is 1mg, and this is the highest available dose in the UK.
Non-formulary medication, for existing patients still taking these medicines.
|
Medication |
Liraglutide Sub-cut (Daily) Non-formulary |
Exenatide Sub-cut (Weekly) Discontinued |
|
Initiation dose |
600 micrograms once daily |
2mg once weekly |
|
Dose titration increment |
600 micrograms once daily |
N/A |
|
Titration interval |
Increase from 0.6mg at 1 week |
N/A |
|
Maximum dose |
Usually 1·2mg once daily Exceptionally 1·8mg/day |
2mg once weekly |
|
Treatment failure criteria |
<11mmol/mol of reduction in HbA1c ±<3% weight loss in 6 months |
|
|
||
|
Renal impairment |
Avoid if eGFR <30mL/min |
Avoid if eGFR <30mL/min |
|
Hepatic impairment |
Avoid in severe hepatic impairment |
Dose as in normal hepatic function |
|
Notes |
|
|
|
||
Clinical review
- HbA1c:
- Reduction of 11mmol/mol (1%)
- OR treatment target of <59mmol/mol met
- Weight: Reduction of 3% of initial weight
Further review
- Consider other factors that may preclude the use of insulin. For example: occupation, social situation and ability to cope with insulin.
Abbreviations
- DPP-4: Dipeptidylpeptidase-4 inhibitor
- GIP/GLP-1 RA: Glucose-dependent insulinotropic polypeptide/Glucagon-like peptide-1 receptor agonist
- GLP-1 RA: Glucagon-like peptide-1 receptor agonist
- MET: Metformin
- PIO: Pioglitazone
- SGLT2: Sodium-glucose co-transporter 2 inhibitor
- SMBG: Self-monitoring of blood glucose
- SU: Sulfonylurea
Editorial Information
Last reviewed: 26/06/2025
Next review date: 30/06/2028
Author(s): Endocrinology (Diabetes Review Group).
Version: 6
Approved By: TAM Subgroup of the ADTC
Reviewer name(s): Dr D MacFarlane, Consultant Endocrinologist..
Document Id: TAM148

